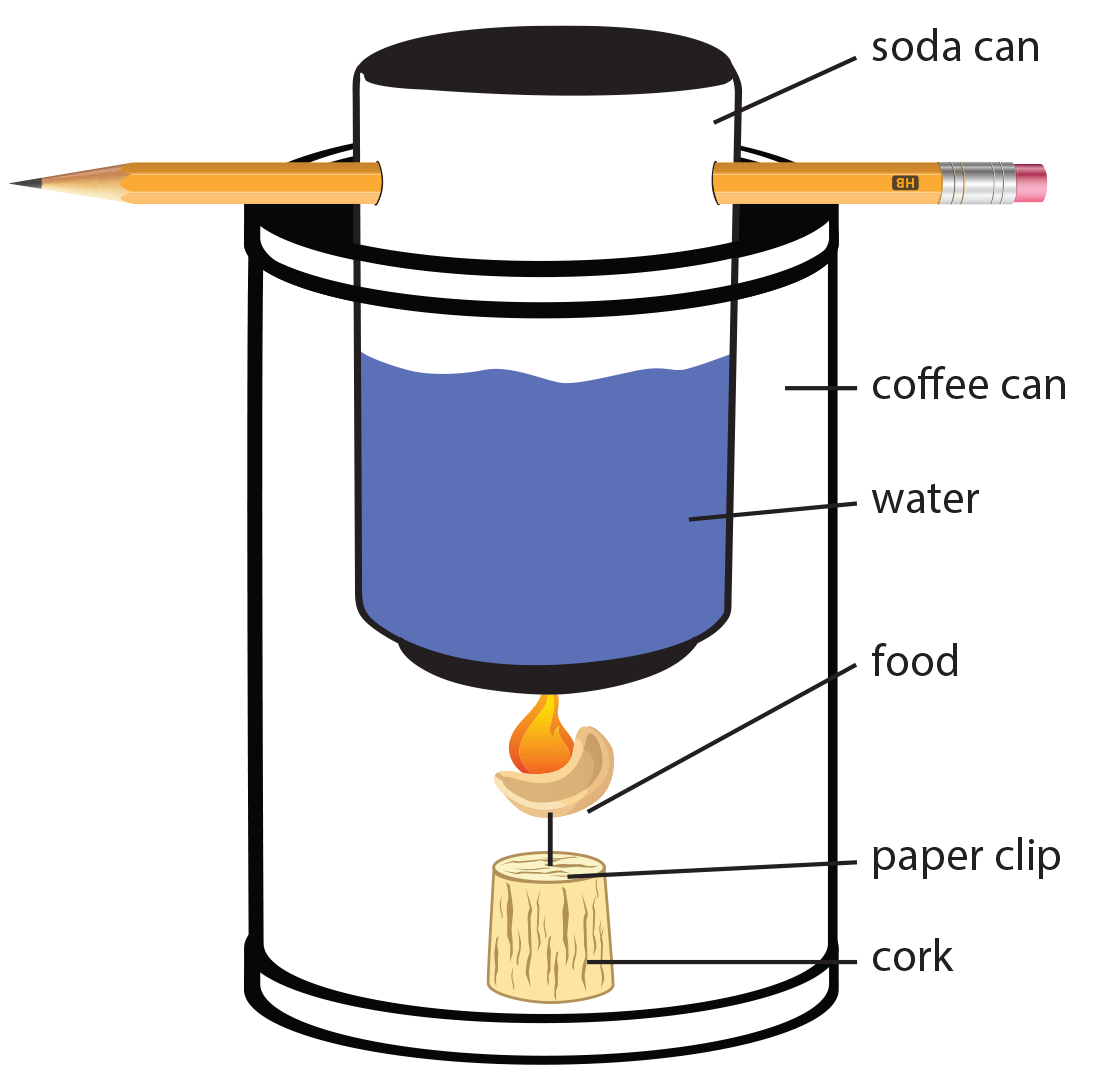
Calorimetry Lab Burning A Peanut . Q peanut = heat produced. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! students will measure the final temperature of the water just as the flame of the peanut fizzles out. Q peanut = m water x 80 cal/g where: food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. the heat produced by the burning peanut is calculated by the equation: The burning of a peanut releases. The bomb calorimeter does a better job of catching all of.
from ksagclassroom.org
The bomb calorimeter does a better job of catching all of. the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. The burning of a peanut releases. Q peanut = m water x 80 cal/g where: Q peanut = heat produced. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. the heat produced by the burning peanut is calculated by the equation: food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. students will measure the final temperature of the water just as the flame of the peanut fizzles out. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut!
FoodMASTER Middle Energy Balance Kansas Agriculture in the Classroom
Calorimetry Lab Burning A Peanut Q peanut = heat produced. Q peanut = heat produced. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. Q peanut = m water x 80 cal/g where: the heat produced by the burning peanut is calculated by the equation: The bomb calorimeter does a better job of catching all of. the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. students will measure the final temperature of the water just as the flame of the peanut fizzles out. The burning of a peanut releases. food scientists use a device known as a bomb calorimeter to measure the calorie content of foods.
From studylib.net
256759 peanutlab Calorimetry Lab Burning A Peanut the heat produced by the burning peanut is calculated by the equation: the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. The bomb. Calorimetry Lab Burning A Peanut.
From ksagclassroom.org
FoodMASTER Middle Energy Balance Kansas Agriculture in the Classroom Calorimetry Lab Burning A Peanut The bomb calorimeter does a better job of catching all of. food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. Q peanut = heat produced. you will calculate the amount of heat. Calorimetry Lab Burning A Peanut.
From www.animalia-life.club
Food Calorimeter Calorimetry Lab Burning A Peanut To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. the heat produced by the burning peanut is calculated by the equation: students will measure the final temperature of the water just as the flame of the peanut fizzles out. The bomb calorimeter does a better job of catching all. Calorimetry Lab Burning A Peanut.
From www.youtube.com
Energy Released from Burning Peanut YouTube Calorimetry Lab Burning A Peanut students will measure the final temperature of the water just as the flame of the peanut fizzles out. the heat produced by the burning peanut is calculated by the equation: Q peanut = m water x 80 cal/g where: Q peanut = heat produced. The burning of a peanut releases. The bomb calorimeter does a better job of. Calorimetry Lab Burning A Peanut.
From www.youtube.com
Peanut Calorimetry video YouTube Calorimetry Lab Burning A Peanut the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. Q peanut = m water x 80 cal/g where: the heat produced by the burning peanut is calculated by the equation: The bomb calorimeter does a better job of catching all of.. Calorimetry Lab Burning A Peanut.
From studylib.net
Student instructions and lab report Calorimetry Lab Burning A Peanut students will measure the final temperature of the water just as the flame of the peanut fizzles out. food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. you will calculate the. Calorimetry Lab Burning A Peanut.
From cetwzrod.blob.core.windows.net
What Is Calorimetry Thermal Physics at Lloyd Dodson blog Calorimetry Lab Burning A Peanut you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! students will measure the final temperature of the water just as the flame of the peanut fizzles out. Q peanut = heat produced. the heat produced by the burning peanut is calculated by the equation: Q peanut =. Calorimetry Lab Burning A Peanut.
From studylib.net
PEANUT and CHEETOE CALORIMETRY LAB Calorimetry Lab Burning A Peanut The burning of a peanut releases. the heat produced by the burning peanut is calculated by the equation: food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! To determine the. Calorimetry Lab Burning A Peanut.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimetry Lab Burning A Peanut the heat produced by the burning peanut is calculated by the equation: food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. The burning of a peanut releases. Q peanut = heat produced. students will measure the final temperature of the water just as the flame of the peanut fizzles. Calorimetry Lab Burning A Peanut.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Burning A Peanut The burning of a peanut releases. the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. Q peanut = m water x 80 cal/g where:. Calorimetry Lab Burning A Peanut.
From www.studocu.com
Energy of a peanut prelab Name___________________ ENERGY OF A PEANUT Calorimetry Lab Burning A Peanut food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. The burning of a peanut releases. To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. the heat evolved by the burning peanut will melt some of the ice and the amount of. Calorimetry Lab Burning A Peanut.
From www.youtube.com
Peanut calorimeter lab instructions and calculations YouTube Calorimetry Lab Burning A Peanut The bomb calorimeter does a better job of catching all of. the heat produced by the burning peanut is calculated by the equation: you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! students will measure the final temperature of the water just as the flame of the. Calorimetry Lab Burning A Peanut.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimetry Lab Burning A Peanut the heat produced by the burning peanut is calculated by the equation: The bomb calorimeter does a better job of catching all of. students will measure the final temperature of the water just as the flame of the peanut fizzles out. Q peanut = m water x 80 cal/g where: the heat evolved by the burning peanut. Calorimetry Lab Burning A Peanut.
From www.gauthmath.com
Solved CalorimetryQuestion 3 A peanut was burned to determine its Calorimetry Lab Burning A Peanut The burning of a peanut releases. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. Q peanut = m water x 80. Calorimetry Lab Burning A Peanut.
From www.numerade.com
SOLVED A student followed the procedure of this experiment determine Calorimetry Lab Burning A Peanut The bomb calorimeter does a better job of catching all of. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. The burning. Calorimetry Lab Burning A Peanut.
From www.scribd.com
Peanut and Cheetoe Calorimetry Lab PDF Calorie Food Energy Calorimetry Lab Burning A Peanut The bomb calorimeter does a better job of catching all of. food scientists use a device known as a bomb calorimeter to measure the calorie content of foods. the heat produced by the burning peanut is calculated by the equation: the heat evolved by the burning peanut will melt some of the ice and the amount of. Calorimetry Lab Burning A Peanut.
From www.vernier.com
Investigating the Energy Content of Foods > Experiment 6 from Calorimetry Lab Burning A Peanut the heat evolved by the burning peanut will melt some of the ice and the amount of liquid water formed will be measured to give the. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! Q peanut = m water x 80 cal/g where: food scientists use. Calorimetry Lab Burning A Peanut.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Lab Burning A Peanut To determine the calorie content of a particular food, its stored bond energy must be liberated and measured. The burning of a peanut releases. you will calculate the amount of heat energy (in joules) and food energy (in calories) contained in a peanut! students will measure the final temperature of the water just as the flame of the. Calorimetry Lab Burning A Peanut.