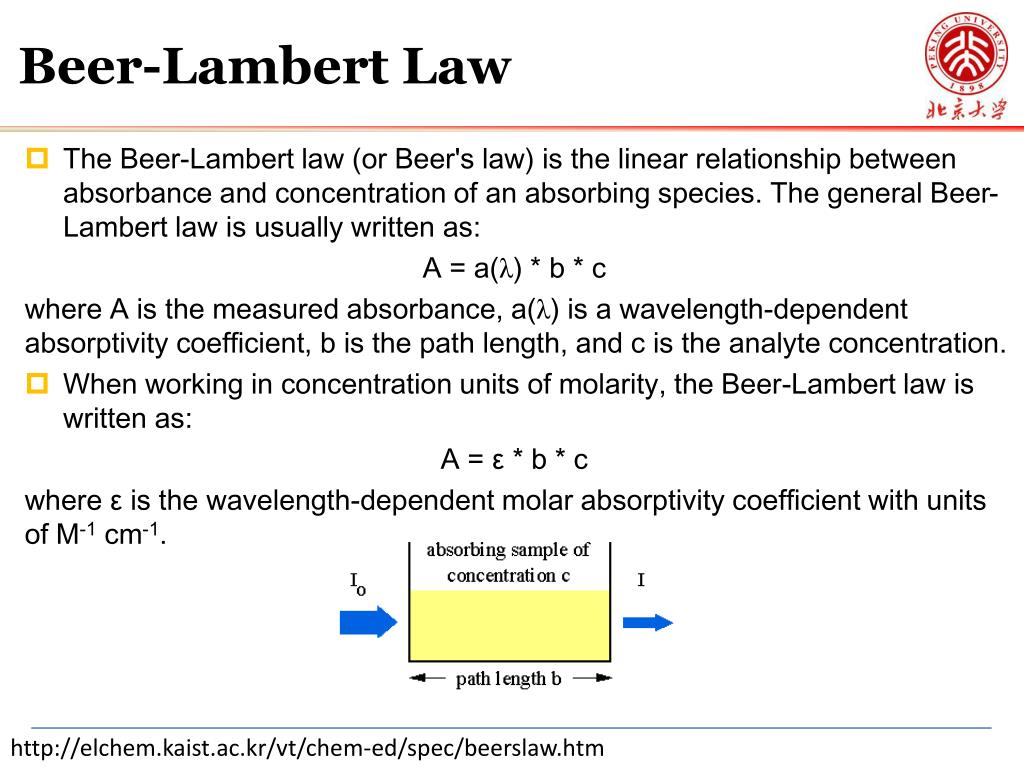
Beer Lambert Law Absorbance . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Ε = molar extinction coefficient. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless.
from www.slideserve.com
It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Ε = molar extinction coefficient. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is.
PPT Absorption and Scattering PowerPoint Presentation, free download
Beer Lambert Law Absorbance since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample.
From exodayzwj.blob.core.windows.net
Beer Lambert Law Units Of Absorbance at John Minor blog Beer Lambert Law Absorbance Ε = molar extinction coefficient. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From egpat.com
Deviations from BeerLambert law Beer Lambert Law Absorbance since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. C = concentration of the sample. Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From studylib.net
Beer Lambert Law Beer Lambert Law Absorbance C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s concentration in a solution and. Beer Lambert Law Absorbance.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Beer Lambert Law Absorbance Ε = molar extinction coefficient. C = concentration of the sample. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Absorbance C = concentration of the sample. Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Absorbance.
From exodayzwj.blob.core.windows.net
Beer Lambert Law Units Of Absorbance at John Minor blog Beer Lambert Law Absorbance \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Ε = molar extinction coefficient. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer Lambert Law Absorbance.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Absorbance C = concentration of the sample. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Ε = molar extinction coefficient. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Absorbance C = concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Ε = molar extinction coefficient. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Absorbance.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Absorbance It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. C = concentration of the sample. Ε = molar extinction coefficient. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Absorbance.
From cienciaydatos.org
Principio de absorción espectroscópica de BeerLambert Beer Lambert Law Absorbance Ε = molar extinction coefficient. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From www.youtube.com
Calculate concentration from UVVis absorbance using BeerLambert's law Beer Lambert Law Absorbance since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Ε = molar extinction coefficient. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. C = concentration of the sample. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Absorbance Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Absorbance.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law Absorbance \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer Lambert Law Absorbance.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Absorbance Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer Lambert Law Absorbance.
From www.slideserve.com
PPT Absorption Spectroscopy of Biopolymers PowerPoint Presentation Beer Lambert Law Absorbance Ε = molar extinction coefficient. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Absorbance since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. C = concentration of the sample. Ε = molar extinction coefficient. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. It provides a mathematical relationship between the substance’s. Beer Lambert Law Absorbance.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Absorbance Ε = molar extinction coefficient. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number. Beer Lambert Law Absorbance.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Absorbance Ε = molar extinction coefficient. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. C = concentration of the sample. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Beer Lambert Law Absorbance.